Lesson 5: States of matter.
Particles in Motion
All matter is made of atoms or groups of atoms that are in constant motion. This idea is the basis for the kinetic theory of matter. How much the particles move and how often they bump into each other determine the state of matter of the substance.
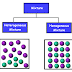
- Solids: Substance that has definite volume and shape. The particles are close together and do not move freely. They vibrate but are fixed in place. Usually particles form a regular pattern.
- Liquids: They have definite volume but not definite shape. Particles have more kinetic energy than particles in a solid. They are attracted to one another and are close together. But they are not fixed in place.
- Gases: They do not have definite volume or shape. Particles have the most kinetic energy of the 3 states. They can move easily in any direction. The space between gas particles can increase or decrease with changes in temperature or pressure.
Properties:Solids: The particles in a solid are in fixed positions and close together. Although the particles vibrate they cannot move from one part of the solid to another part. Solids cannot easily change shape or volume. You can change the shape of a solid by breaking it into pieces. However, each piece will still be a solid and have its own definite shape.Liquids: All liquids have definite volume but no definite shape. The particles in a liquid are close together but not tightly attached to one another. They can slide past each other. That´s why liquids can flow. They take the shape of their container.Gases: They do not have definite volume or shape. The particles are very far apart. And the amount of space between them can change more easily.






















0 comments:
Post a Comment